Cross-Linking FAQ: How Painful is iLink® FDA-Approved Cross-Linking?
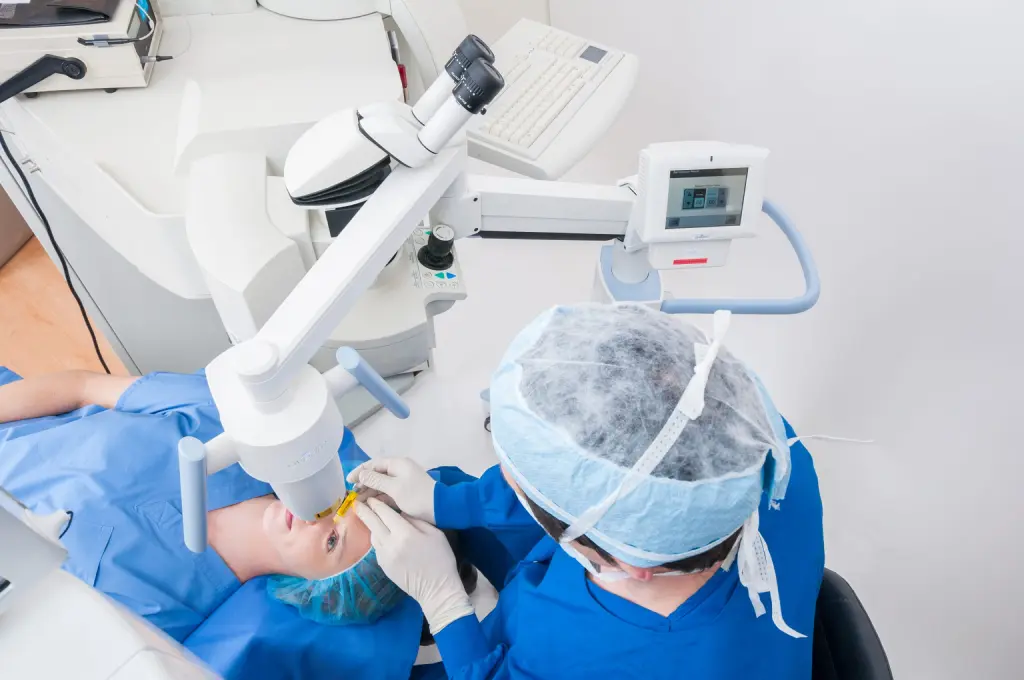
With any medical procedure, one of the first questions that comes to mind is “Is it painful?” Cross-linking is no exception. If you or someone you know is considering getting iLink® FDA-approved cross-linking to treat progressive keratoconus and wondering if the procedure is painful, you are certainly not alone. This is an important question to be asking!
iLink is a minimally-invasive treatment proven to be safe and effective in slowing or halting the progression of keratoconus to preserve vision. During the procedure, the epithelium (the thin layer on the surface of the cornea) is gently removed and Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) eye drops will be applied to the cornea for at least 30 minutes. Depending on the thickness of the cornea, Photrexa® (riboflavin 5’-phosphate ophthalmic solution) drops may also be required. Finally, the cornea is exposed to UV light for 30 minutes while additional Photrexa Viscous drops are applied.
We know everyone’s experiences are different, but we want to help you feel as prepared and as comfortable as possible going into your procedure. Below, we’re discussing potential pain associated with iLink FDA-approved cross-linking. Continue reading to learn more and listen to someone in the KC community discuss her recovery experience.
Does the Procedure Hurt?
iLink FDA-approved cross-linking is a minimally invasive surgery. During the procedure, you may be given medication to help you relax and anesthetic drops to help numb the pain. With iLink, the only cross-linking procedure that is approved by the FDA, there can be irritation during the immediate recovery but usually not during the treatment. Immediately following the treatment, a bandage contact lens is placed on the surface of the eye to protect the area. After the numbing drops wear off, you may experience some discomfort, often described as a gritty, burning sensation. This discomfort can be managed with Tylenol, artificial tears, and resting ice packs on your eyes. If your pain is severe, make sure to discuss this with your doctor, as oral narcotic medications may be used. Although undergoing a minimally invasive surgery might seem intimidating, discuss with your doctor the disadvantages of waiting too long. Choosing to wait and allowing your condition to progress could potentially cause the need for a corneal transplant in the future, which is why your doctor may recommend to act now.
Hear From a KC Doctor

If you’re looking for more information on what to expect following your iLink procedure, discuss the risks and benefits of the procedure with your doctor. You can also hear from one of our KC doctors. Dr. Neda Shamie, a cataract, LASIK and corneal surgeon and partner at Maloney-Shamie Vision Institute in Los Angeles, California, who shares more on the matter below:
“The recovery is often quick, and the most symptomatic period typically is in the first few days after the procedure, while the epithelium is healing. Patients are given pain medications and a sleeping pill for the first night to help them through the first 24 hours when the discomfort may be at its worse, albeit tolerable. Patients describe feeling as though they have a large scratch on their eye although the bandage contact lens does help alleviate much of the discomfort. As with all post-surgical care, patients need to be seen frequently until the surface epithelium heals and to be monitored for possible signs of infection. I typically see the patient at the post-op day one and again three days later to remove the contact lens and ensure that, as is usually the case, the epithelial defect is fully healed. The vision may fluctuate over the first month while the cornea is remodeling but stabilization and flattening is appreciated after about 3 months.”
Bekah Shares Her Experience
If you’ll feel most comfortable listening to someone within the keratoconus community who has already undergone FDA-approved cross-linking, look no further. After deciding to receive iLink cross-linking, Bekah video blogged her journey before and after the procedure to share her experience with others. Watch Bekah’s Video Journal below.
Remember to discuss iLink with your doctor to determine if it may be right for you. If you’re interested, you can also connect with others in the keratoconus community who may be going through a similar experience.
Don’t forget to follow us on Facebook, Twitter, and Instagram for more information on keratoconus and iLink FDA-approved cross-linking.