Blogs
Real and timely KC information
Blog entries from people with KC and doctors alike share the latest news, expert perspectives, and management strategies that can be helpful lifelines for your day-to-day experience of KC.

-

Your Guide to Recovering from iLink® FDA-Approved Cross-Linking
Read more: Your Guide to Recovering from iLink® FDA-Approved Cross-LinkingIt’s important to know that recovery is different for everyone. We’re providing you with a full iLink® Recovery Guide below, which includes information on how to prepare for your procedure, when you can expect to return to your normal routine, and suggested essentials. Continue reading to learn more!
-
Cross-Linking FAQ: How Painful is iLink® FDA-Approved Cross-Linking?
Read more: Cross-Linking FAQ: How Painful is iLink® FDA-Approved Cross-Linking?With any medical procedure, one of the first questions that comes to mind is “Is it painful?” Cross-linking is no exception. If you or someone you know is considering getting iLink® FDA-approved cross-linking to treat progressive keratoconus and wondering if the procedure is painful, you are certainly not alone. This is an important question to…
-

Summer Vacation: An Ideal Time to Undergo Corneal Cross-Linking
Read more: Summer Vacation: An Ideal Time to Undergo Corneal Cross-LinkingFor people living with keratoconus, a progressive eye disease in which the normally round cornea thins and begins to bulge into…
-

Should You Seek a Second Opinion Before Receiving Cross-Linking?
Read more: Should You Seek a Second Opinion Before Receiving Cross-Linking?If you’ve never asked for a second opinion before, the decision can feel overwhelming, so we’re here to help. Read our blog to determine if you should receive a second opinion on your recommended keratoconus treatment options.
-

The Importance of Receiving iLink® FDA-Approved Cross-Linking
Read more: The Importance of Receiving iLink® FDA-Approved Cross-LinkingiLink® FDA-approved cross-linking is the only treatment option proven safe and effective in slowing or halting the progression of keratoconus to help preserve patients’ vision.
-

Video Journal: One Woman Shares Her Cross-Linking Journey
Read more: Video Journal: One Woman Shares Her Cross-Linking JourneyBekah was diagnosed with keratoconus when she was 36 after she failed her vision exam at the DMV. Similar to others who are diagnosed with this condition, Bekah didn’t know anyone who was living with keratoconus.and began a quick study of the condition and her potential treatment options.
-
Cross-Linking FAQ: How Long Does it Take to Recover From iLink®?
Read more: Cross-Linking FAQ: How Long Does it Take to Recover From iLink®?If you or someone you know has keratoconus, it is likely that you have heard of iLink® FDA-approved corneal cross-linking. When it comes to iLink®, we want to help answer as many of your questions as possible, including how long it takes to recover from FDA-approved cross-linking and what that process may look like for…
-

Cross-Linking FAQ: Does Corneal Cross-Linking Help Vision?
Read more: Cross-Linking FAQ: Does Corneal Cross-Linking Help Vision?One of the more common questions that we’ve seen from the Living with Keratoconus community is: Will corneal cross-linking help my vision? In this blog, we’re discussing whether iLink® FDA-approved cross-linking helps improve vision, and if corrective lenses will be needed.
-

How I’m Managing My Keratoconus and Why I Decided Against a Corneal Transplant
Read more: How I’m Managing My Keratoconus and Why I Decided Against a Corneal TransplantI was in my early 30s when I first learned I was living with keratoconus, but the journey to finding a treatment option that was right…
-

Unapproved Cross-Linking Failure Leads to Vision Loss
Read more: Unapproved Cross-Linking Failure Leads to Vision LossThere is only one FDA-approved procedure proven to be safe and effective in slowing or halting keratoconus progression to help preserve a person’s vision: iLink®. Any other corneal cross-linking procedures are unapproved, which means that NOT ALL corneal cross-linking treatments are the same.
-

Cross-Linking FAQ: How Successful is iLink® FDA-Approved Cross-Linking?
Read more: Cross-Linking FAQ: How Successful is iLink® FDA-Approved Cross-Linking?We know being diagnosed with a progressive condition can be daunting, and you likely have a lot of questions. To help, we’re discussing the efficacy of FDA-approved cross-linking and sharing how some people in the Living with KC community are doing after receiving this procedure which preserves sight for many of the recipients.
-
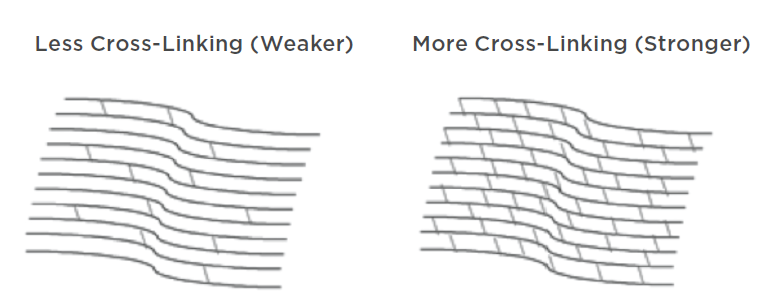
Epi-Off, Epi-On, C3R – What Does It All Mean?
Read more: Epi-Off, Epi-On, C3R – What Does It All Mean?If you or a family member has been diagnosed with keratoconus, then you’ve probably read about corneal cross-linking as a potential treatment option.
-

Understanding Insurance Coverage for Cross-Linking in the U.S.
Read more: Understanding Insurance Coverage for Cross-Linking in the U.S.Insurance coverage for cross-linking is a hot topic among the keratoconus community. Understanding all of the specifics can be confusing. In this blog, we answer your most frequently asked questions.
-
Curious About Insurance Coverage for Cross-Linking? Here’s What to Know!
Read more: Curious About Insurance Coverage for Cross-Linking? Here’s What to Know!We all know that health insurance coverage can be a pain to navigate, regardless of the situation! To help clear up any confusion, we’re highlighting everything you need to know about insurance coverage for iLink® FDA-approved cross-linking.
-

KC Patient and Doctor Perspectives: The Importance of Early Diagnosis and Corneal Cross-Linking
Read more: KC Patient and Doctor Perspectives: The Importance of Early Diagnosis and Corneal Cross-LinkingFor keratoconus patient Kiana, attaining an early diagnosis and corneal cross-linking proved crucial to managing her condition long-term.
-

Recently Diagnosed with Keratoconus? We’ve Got You Covered!
Read more: Recently Diagnosed with Keratoconus? We’ve Got You Covered!Despite the fact that an estimated 253 million people in the world live with vision impairment, learning that you or a loved one has vision issues can feel isolating and overwhelming.
-

Keratoconus 101: Treatment Options and Post-Treatment Care
Read more: Keratoconus 101: Treatment Options and Post-Treatment CareIf untreated, keratoconus may result in significant vision loss and can lead to a corneal transplant in severe cases. However, there are various FDA-approved treatment options available that work to treat the symptoms caused by the thinning and bulging of the cornea.