About iLink®
A treatment to slow or halt the progression of keratoconus
The first and only cross-linking procedure that’s FDA-approved, iLink is a minimally invasive outpatient procedure.

Don’t risk your vision on a procedure that does not have a proven safety profile. If your keratoconus is not effectively treated and continues to progress, you may lose vision that cannot be recovered.
Your sight is extremely important, and therefore deserving of a safe procedure, such as iLink.
How it works
During the cross-linking procedure, the combination of UV light and eyedrops helps to stiffen and strengthen the collagen fibers of the cornea that have been weakened by the disease. Overall, the goal of cross-linking is to slow or halt progressive keratoconus to preserve vision. In most cases, the procedure takes about an hour, and you can return to the comfort of your own home the same day.
Watch WiseEyes – The iLink® cross-linking procedure
Learn more about how the only FDA-approved cross-linking procedure works by strengthening and stiffening the cornea to slow or halt the progression of keratoconus.
First and only
FDA-approved
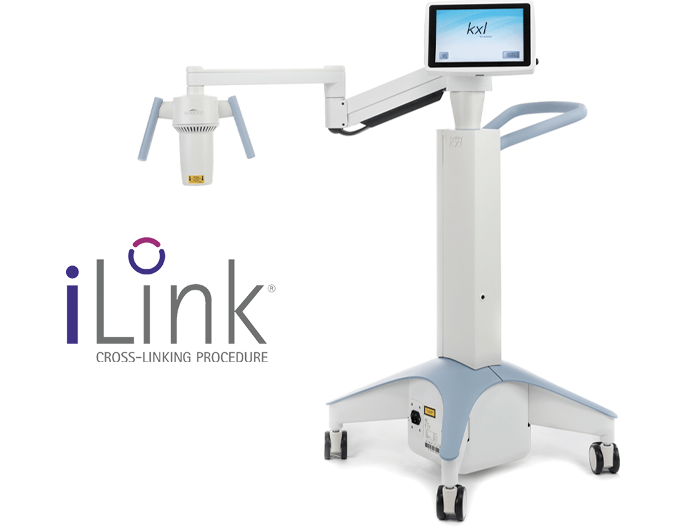
iLink is the first and only FDA-approved cross-linking treatment, combining the use of prescription eyedrops, Photrexa®️ Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution), Photrexa®️ (riboflavin 5’-phosphate ophthalmic solution), and ultraviolet (UV) light from the KXL® system to create new collagen cross-links.
Why does FDA approval matter?
Without tightly controlled, randomized clinical trials, it is not possible to tell the actual success rate and potential complications of a procedure. The Food and Drug Administration (FDA) requires such trials, and closely analyzes the results, before granting approvals. For unapproved drugs and devices, the FDA has not determined that they are safe and effective.
Ask your doctor
Is the cross-linking they perform FDA-approved and covered by insurance?
What is the difference between iLink and non–FDA-approved procedures?
What is epi-off cross-linking?
What is epi-on cross-linking?
What is Holcomb C3R®?
iLink is the only FDA-approved cross-linking procedure in the United States
GET THE BROCHURE
ENGLISH

SPANISH / ESPAÑOL

Going for your iLink procedure?
Need help finding a corneal specialist?
REFERENCES
- Pramanik S, Musch DC, Sutphin JE, Farjo AA. Extended long-term outcomes of penetrating keratoplasty for keratoconus. Ophthalmology. 2006;113(9):1633-1638.
- Maharana PK, Agarwal K, Jhanji V, Vajpayee RB. Deep anterior lamellar keratoplasty for keratoconus: a review. Eye Contact Lens. 2014;40(6):382-389.