Your Child Has Been Diagnosed with Keratoconus…Now What?
As a parent, you always want what’s best for your child and you want them to be healthy. While any medical news or diagnosis may come as a shock, oftentimes there are options available to treat or manage the condition. This is true for keratoconus. If you’ve noticed that your teenager is experiencing some issues with his or her vision without any explanation behind it, keratoconus may be a scary, but ultimately welcomed diagnosis.
Since keratoconus is a genetic[1] [2] condition, it may not be a total surprise if other family members are also living with this progressive condition. If your teenager was diagnosed with progressive keratoconus, the condition was hopefully detected early.
Below we’re answering some commonly asked questions, sharing tips on how to communicate with your child about their diagnosis, and detailing firsthand accounts from parents who have gone through this process themselves.

Do You Have Questions? We Have the Answers!
You likely want to learn everything you can about your teenager’s recent keratoconus diagnosis. Maybe you have never heard of the condition before and want more information. Don’t worry, we’ve got you covered!
What is keratoconus?
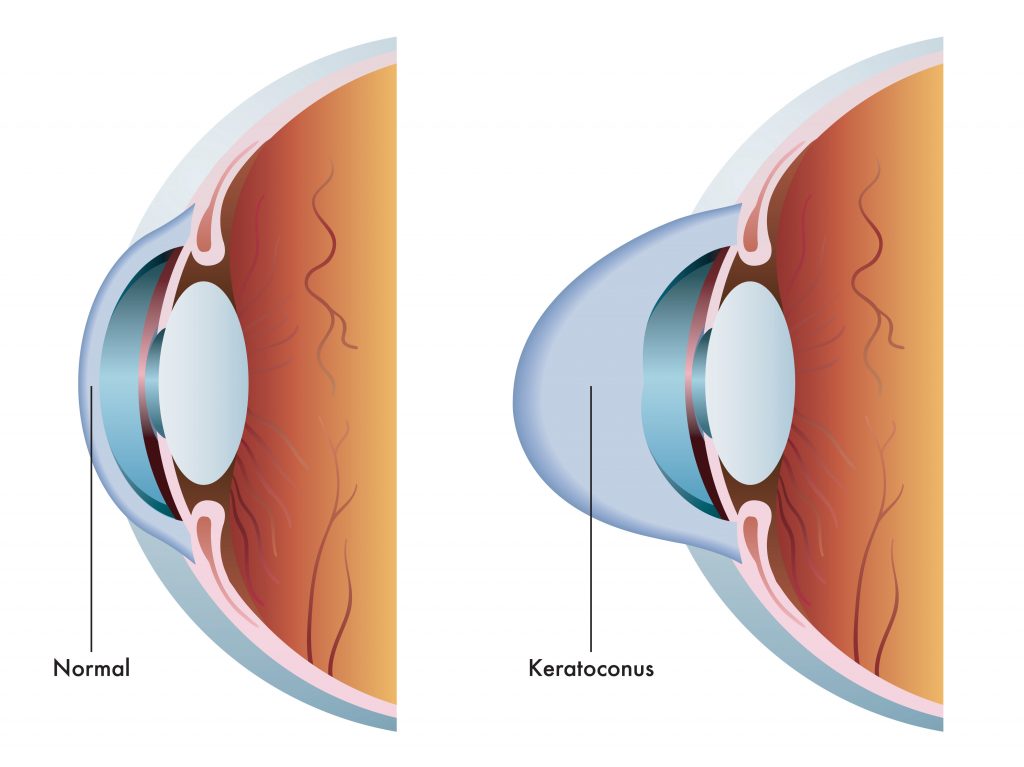
Keratoconus, often referred to as ‘KC’, is an eye condition in which the cornea weakens and thins over time, causing the development of a cone-like bulge and optical irregularity of the cornea. The condition can result in significant visual loss and may lead to corneal transplant in severe cases. The condition typically first appears in individuals who are in their late teens or early twenties, and may progress for 10-20 years, and then slow or stabilize. Each eye may be affected differently, and in the early stages of keratoconus, people might experience slight blurring of vision, distortion of vision, and increased sensitivity to light.
What keratoconus disease intervention and vision management options are available?
Disease intervention consists of halting or slowing disease progression. Vision management focuses on correcting the distorted vision caused by the thinning and bulging of the cornea. End-stage surgery is a last resort option.
- Vision Management: Eyeglasses or Soft Contact Lenses, Rigid Gas Permeable Contact Lenses, and Intacs
- Intervention – To halt or slow disease progression: Corneal Cross-Linking
- End-Stage Surgery – To replace severely diseased or scarred corneal tissue with healthy tissue from an organ donor: Corneal Transplant Surgery
What questions should I be asking the eye doctor?
Prepare for your next appointment by creating a list of questions you can ask to help you navigate through your family’s keratoconus journey. Not sure what to ask, considering some of the following questions:
- How will keratoconus affect my teenager’s vision?
- Will their keratoconus get worse?
- What are the available treatment options, and which do you recommend?
- How can keratoconus be stopped from progressing?
- Is the procedure that you are recommending FDA approved or investigational?
- Is there anything that makes keratoconus worse?
- Is keratoconus genetic? Should I make sure my family members get tested?
You can read the full list of questions here.
Should I get screened for keratoconus? Should I have my other children screened?
People with a parent, sibling, or child who has keratoconus have a 15 to 67 times higher risk of developing corneal ectasia compared to patients with no affected relatives[4] [5]. If your teenager has been diagnosed with keratoconus, there is a possibility that others in your family may also be living with the progressive condition as well. Don’t wait to make an appointment for a comprehensive eye exam.
How to Talk About a Keratoconus Diagnosis with Your Teenager

Keratoconus typically first appears in individuals who are in their late teens or early twenties. However, there have been instances where young children have also been diagnosed. The age of your child will determine how you go about discussing their diagnosis with them. For example, a high school student or young adult may have a better understanding of the condition based on what the physician has explained to them and their own research. On the other hand, a young child who was diagnosed with a vision issue may not really understand what is happening, what might need to change (ex: contacts), or what this will mean for them [6].
Below are some tips to consider when discussing a keratoconus diagnosis with your child.
Educate yourself first
After a doctor tells you about your teenager’s condition, you might feel overwhelmed. The doctor likely shared a lot of information and new terms that can be difficult to process all at once. Take the time to review all of the information provided and do your research. With this information, you will be better able to explain the situation to your teenager and answer any questions that they may have. It may also lead to some follow-up questions that you want the physician to clarify at the next appointment.
Speak with your teenager
Following a diagnosis, talk with your teenager to make sure they understand what is going on and give them the chance to ask any questions they may have after their appointment. While your child might be confused, sharing the benefits of early diagnosis and available treatment options in a way they can understand can help to ease any uncertainty. As a team, you can determine the best plan to preserve their vision in consultation with a physician.
Let them know that they are not alone
Being diagnosed with a health condition can be an isolating experience, but it doesn’t have to be. You can help your teenager feel connected and supported by assuring them that you will be there to help every step of this journey. While their friends may not be living with keratoconus, some may also have vision issues and need to wear glasses or contact lenses, or could be dealing with other health issues. You can also help them find a way to talk about their diagnosis and what it means in a way that is easy for their peers to understand.
Connect with Support Groups
Although it may not seem like it, you are a big part of your teenager’s keratoconus journey. This may mean that at times you will also need some support. There are support groups that parents and young adults can join to connect with others who are living with keratoconus, including the National Keratoconus Foundation and the Keratoconus Group. In these groups, people come together to look for information, ask for help, share their personal experiences or those of their loved ones, or find comfort in reading what others are going through. Below are two parents* in the Living with KC community who shared what it was like for their teenagers to be diagnosed with the progressive condition – including one mother who is also living with the condition.

Pamela: As a mother of three boys, one of Pamela’s biggest concerns is their health. When her two oldest boys started complaining of vision problems, she suspected right away what might be causing their issues, as she and her sister had been living with keratoconus for many years. Pamela is incredibly thankful that her boys may not have to go through what she did and she is confident that keratoconus won’t consume their lives or futures.

Mat: In the first grade, Mat’s son Luke began complaining of vision issues, such as blurriness and double vision. To help correct this, Luke was prescribed glasses, but over the next five to six years, his vision continued to decline. After getting a second opinion, Mat felt immense relief to finally receive an accurate diagnosis for his son – he was living with keratoconus and referred to a local ophthalmologist.
*Results may vary
Looking Forward to the Future
Remember you and your child are not alone. There are many people in the keratoconus community going through the same thing as your family. We understand that a keratoconus diagnosis is difficult to process, but thanks to technological advances, there are treatments available to help slow or halt the progression of the disease and preserve vision.
Making sure you are informed with the right information is the first step to finding a physician and treatment path that is right for your teenager. For more information on keratoconus visit our website. To find a doctor near you to get screened for keratoconus, use our locator tool.
Resources
[1] Fecarotta CM, Huang WW. Pediatric genetic disease of the cornea. J Pediatr Genet. 2014;3(4):195–207. doi:10.3233/PGE-14102
[2] Wang Y, Rabinowitz YS, Rotter JI, Yang H. Genetic epidemiological study of keratoconus: evidence for major gene determination. Am J Med Genet. 2000;93(5):403–409
[4] Fecarotta CM, Huang WW. Pediatric genetic disease of the cornea. J Pediatr Genet. 2014;3(4):195–207. doi:10.3233/PGE-14102
[5] Wang Y, Rabinowitz YS, Rotter JI, Yang H. Genetic epidemiological study of keratoconus: evidence for major gene determination. Am J Med Genet. 2000;93(5):403–409
[6] Ferdi AC, Nguyen V, Gore DM, Allan BD, Rozema JJ, Watson SL. Keratoconus Natural Progression: A Systematic Review and Meta-analysis of 11 529 Eyes. Ophthalmology. 2019 Jul;126(7):935-945. doi: 10.1016/j.ophtha.2019.02.029. Epub 2019 Mar 8. PMID: 30858022.