Videos, Blogs, and Journeys
Videos
There are others out there who’ve been through what you may be experiencing now. The perspective of KC patients, caretakers, and doctors can help.
-
Sylvia & Sarah discuss their journey to a keratoconus diagnosis – 1 of 4
-
Learning about Keratoconus – 2 of 4
-
Sylvia discusses Down syndrome, Keratoconus and spreading awareness – 3 of 4
-
Looking towards Sarah’s future & the importance of vision – 4 of 4
BLOGS
Blogs feature the latest news, professional opinions, and tips helpful for your daily life with KC.
-

Taking the Plunge: Shradha’s Journey to the Depths of the Ocean with Keratoconus
Read more: Taking the Plunge: Shradha’s Journey to the Depths of the Ocean with KeratoconusBy: Shradha I was diagnosed with keratoconus at a time in my life when I was enthusiastic about changing the world. What I ended up changing, however, were seven prescriptions and five doctors over the course of two years, before a specialist finally diagnosed my condition. These were not the only changes I was dealing […]
-
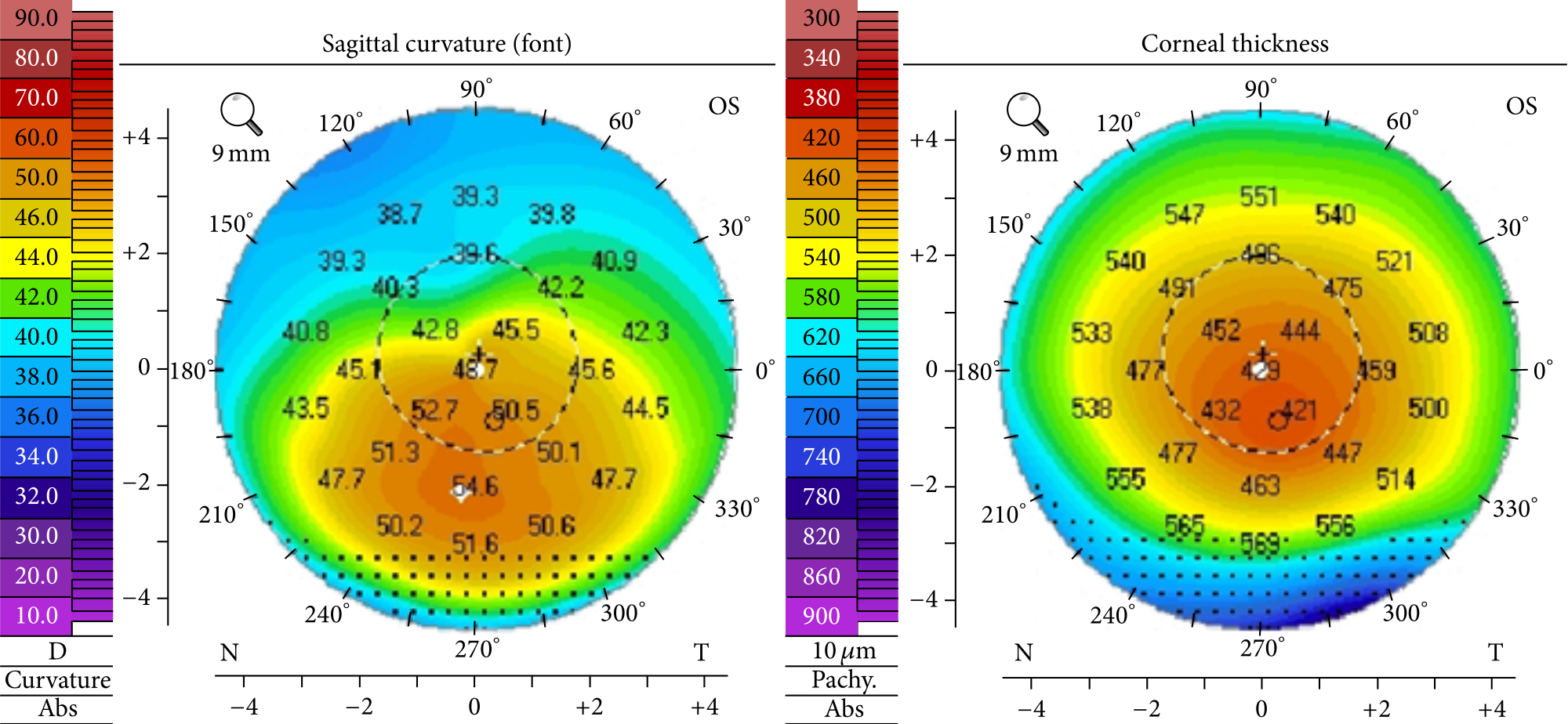
What To Know About Corneal Topography – Part 1
Read more: What To Know About Corneal Topography – Part 1Whether you’ve worn glasses or contacts for years or not, realizing your vision is declining can be a scary experience. For patients suspected to have keratoconus, doctors may order multiple tests with corneal topography.
-

What To Know About Corneal Topography – Part 2
Read more: What To Know About Corneal Topography – Part 2Now that we’ve talked about how keratoconus is diagnosed and what corneal topography is, it’s time to focus on what to expect during the testing process.
-

Sports Illustrated: Golden State Warrior Steph Curry Wears Contacts to Address Eye Condition
Read more: Sports Illustrated: Golden State Warrior Steph Curry Wears Contacts to Address Eye ConditionIn April 2019, Stephen (Steph) Curry, an American professional basketball player for the Golden State Warriors, announced that he is living with keratoconus…
Journeys
The KC community is ready for you with information from doctors, caretakers, and people living with keratoconus.
-

Ash’s Keratoconus Journey
Read more: Ash’s Keratoconus JourneyMore than 317K people follow Ash Arellanes – @demi_the_vlog_dog – on TikTok because of the spirited, engaging, and informative video content she shares about her journey with keratoconus (KC), a progressive eye disease. It’s hard to imagine this 27-year-old young woman could barely see just six years ago. Now, she is committed to spreading awareness for this underdiagnosed, sight-threatening eye condition.
-

A Father’s Perspective: Mat
Read more: A Father’s Perspective: MatIn the first grade, my son Luke began complaining of vision issues, such as blurriness and double vision. To help correct this, we brought him to an optometrist who prescribed him glasses to help him see. What my wife and I didn’t realize was that our son was living with a progressive eye disease known as keratoconus.
-

Sarah’s Video Series: A Story of Keratoconus, Down Syndrome, and Treatment
Read more: Sarah’s Video Series: A Story of Keratoconus, Down Syndrome, and TreatmentDuring their initial appointment, the corneal specialist explained that 5-15% of people with Down syndrome also have keratoconus and recommended that Sarah consider FDA-approved corneal cross-linking…
-

My Family’s Journey with Keratoconus
Read more: My Family’s Journey with KeratoconusGrowing up, I thought I had perfect vision. I didn’t wear glasses or contacts and my doctors never noticed anything problematic with my eyes…